Introduction
Perfluorobutanesulfonic acid (PFBS) is one of many synthetic chemicals in the PFAS family, widely used as a replacement for PFOS following its phase-out. Marketed as a ‘safer’ alternative due to its shorter biological half-life in humans, PFBS has since come under regulatory scrutiny as studies reveal its persistence in the environment and potential health risks. This article explores how PFBS is used, how it spreads through ecosystems, its health impacts, evolving regulations, and steps individuals can take to reduce exposure. This article explores how PFBS is used, how it spreads through ecosystems, its health impacts, evolving regulations, and steps individuals can take to reduce exposure.
Background on PFBS
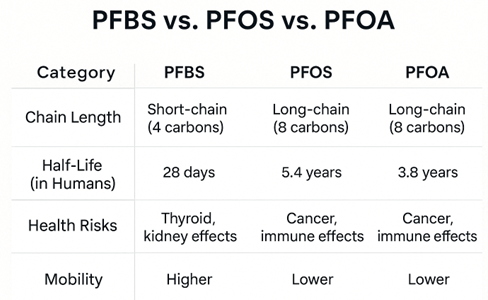
PFBS is a short-chain PFAS compound made up of four fully fluorinated carbon atoms attached to a sulfonic acid group. Its small molecular structure makes it more water-soluble than long-chain PFAS like PFOS and PFOA. These traits help PFBS travel farther in groundwater and surface water.
Why was PFBS Introduced?
Introduced in the early 2000s, PFBS was part of the industry’s shift away from long-chain PFAS amid mounting evidence of toxicity. Its shorter human half-life (around 30 days) was seen as an improvement over PFOS and PFOA, which persist in the body for years. However, PFBS is still highly persistent in the environment and difficult to remove from drinking water. Its solubility increases the risk of contamination, even in areas with no direct PFBS use. In response, regulators have begun setting health-based drinking water guidelines and are increasingly supporting class-based PFAS regulation rather than evaluating each compound in isolation.
PFBS Uses and Applications
PFBS is used in a variety of commercial and industrial applications, including:
- Stain- and water-resistant textiles: PFBS is used to treat fabrics in clothing, upholstery, and carpets to repel water, oil, and dirt.
- Firefighting foams (AFFF): Aqueous film-forming foams containing PFBS are used in high-risk areas like airports, military bases, and chemical plants to suppress fuel-based fires.
- Cleaning products and polishes: PFBS is included in surface coatings to maintain shine and resist dirt. It helps products spread evenly and maintain gloss without degrading under heat or chemical stress.
- Food packaging coatings: PFBS is used to coat wrappers and containers to prevent grease and moisture soak-through.
- Semiconductor manufacturing: PFBS is used in photolithography due to its stability under high temperatures and resistance to chemical breakdown during microchip production.
PFBS Contamination in Water
Due to its widespread use and high mobility in water, PFBS contamination has been detected across a range of environmental and indoor settings.
- Municipal drinking water supplies near fluorochemical plants
PFBS has been found in public water systems located near facilities that manufacture or use fluorinated compounds. These plants often release PFBS through industrial wastewater, which can contaminate nearby aquifers and rivers used for drinking water. - Groundwater beneath airports and military installations
Sites that historically used AFFF (aqueous film-forming foam), including military bases and commercial airports, have reported PFBS in monitoring wells and groundwater. These foams were often applied directly to the ground during training and emergency response. - Surface waters near wastewater discharge points
Municipal wastewater treatment plants are not equipped to remove PFBS effectively, so treated effluent can carry it into rivers and lakes. PFBS may enter sewer systems from household products or industrial waste.
A 2023 U.S. Geological Survey study found PFBS in surface water, groundwater, and tap water across multiple states, even in locations far from known industrial sources. It was also detected in human blood samples, confirming ongoing exposure from both environmental and consumer-based pathways.
Human Exposure to PFBS
The most common routes of PFBS exposure include:
- Drinking contaminated water. PFBS has been detected in municipal and private drinking water supplies, especially near fluorochemical manufacturing facilities and areas where aqueous film-forming foams (AFFF) were used.
- Eating food packaged in grease-resistant wrappers or boxes. PFBS can migrate into food from grease-resistant packaging materials, such as fast-food containers and microwave popcorn bags.
- Inhaling or ingesting indoor dust containing PFBS from treated surfaces. PFBS-treated products like carpets and upholstery can shed particles into indoor dust, which may be inhaled or ingested, particularly by young children.
- Dermal contact with products that contain PFBS, such as stain-proof coatings. While dermal absorption of PFBS is generally low, repeated skin contact with PFBS-containing products may contribute to overall exposure.
Communities living near former or active military bases, chemical plants, and landfill sites face the highest risk where residents regularly ingest contaminated water from local sources.
Occupational exposure is also a concern for firefighters, industrial workers, and wastewater treatment personnel, though transdermal exposure has been less of a concern in current scientific studies.
Testing for PFBS in Drinking Water
PFBS is not as widely monitored as PFOS or PFOA, but some state and local agencies have begun to include it in routine water testing programs. In the U.S., states like Michigan, California, and Minnesota have added PFBS to their list of regulated PFAS in public water systems.
The testing methods used to detect PFBS are similar to those for other PFAS and include EPA Method 537.1 and Method 533. These involve collecting water samples and analyzing them using liquid chromatography coupled with mass spectrometry (LC-MS/MS). Certified labs can detect PFBS concentrations at parts-per-trillion (ppt) levels.
Households can request water testing through municipal utilities or certified labs. For those who receive a positive result, water filtration systems that use activated carbon or reverse osmosis have been shown to reduce PFBS levels effectively.
Epidemiological Studies with PFBS
One of the key concerns with PFBS is the limited availability of long-term epidemiological studies in humans. While animal studies have raised red flags about thyroid disruption, liver toxicity, and reproductive issues, equivalent long-term studies in humans are lacking. This gap in the research makes it difficult for regulators and scientists to fully quantify the risks posed by chronic, low-level PFBS exposure.
Because PFBS has a shorter half-life in the human body than long-chain PFAS, it was assumed to pose less of a threat. However, emerging research indicates that repeated exposure, even to a compound with a short half-life, can still cause harm, especially when environmental contamination is persistent.
The Need for Class-Based PFAS Regulation
The regulatory framework around PFAS has traditionally taken a compound-by-compound approach, assessing each PFAS chemical individually. This method is slow and ineffective, given the sheer number of known PFAS (over 12,000), including variants like PFBS.
Experts are now advocating for class-based regulation, that would treat all PFAS as a group, unless proven safe. This approach is supported by scientific advisory panels in the U.S. and abroad.
By regulating PFAS as a class, lawmakers can close loopholes that allow manufacturers to simply substitute one compound for another without proper safety evaluations. This shift could prevent harmful replacements like PFBS from gaining widespread use before their risks are fully understood.
Public Health Messaging Around PFBS
As awareness of PFBS grows, public health agencies have begun tailoring educational materials to communicate its potential risks. While PFBS may be less bioaccumulative than long-chain compounds like PFOS and PFOA, it remains environmentally persistent and toxic at low levels.
Agencies recommend actions such as reading water quality reports, avoiding stain-resistant products, and using certified PFAS filters. These community-focused strategies are designed to empower individuals while driving broader public support for PFAS regulation.
Conclusion
PFBS was introduced as a safer alternative to legacy PFAS like PFOS and PFOA, but it has brought its own set of concerns. While it may not remain in the human body as long, PFBS is highly persistent in the environment and has been detected in water supplies, wildlife, and everyday consumer products. Emerging research suggests potential health risks, particularly for vulnerable populations.
These realities highlight the limitations of one-to-one chemical substitution and the need to shift toward class-based PFAS regulation. As scientific understanding of PFBS and related compounds continues to grow, regulatory approaches must evolve accordingly, from reactive bans to precautionary, science-driven policies.
By staying informed, advocating for transparency, and supporting protective regulations, individuals and communities can play a meaningful role in reducing PFAS exposure and pushing for long-term environmental health reforms. Continued investment in research, transparent labeling of consumer products, and strict enforcement of water safety limits will be essential in protecting public health.
Written By:

Dr. Francis Okejiri
Scientific Contributor
Dr. Francis Okejiri is a scientific author and reviewer at PFAS Water Experts. He holds a PhD in chemistry with a focus in analytical and environmental science. He has authored over 17 peer-reviewed publications and brings broad experience in evaluating contaminants and complex environmental data.